LAL Reagent Vials for Endotoxin Detection Kinetic Chromogenic Method
Min.Order : 1 Pieces Quick Quotation >
Item Details
Product Description
LAL reagent vials for endotoxin detection kinetic chromogenic method
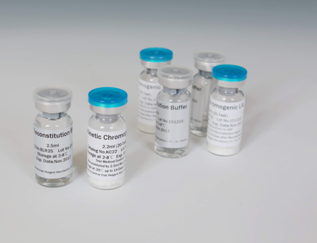
LAL reagent vials for endotoxin detection kinetic chromogenic method
We supply kinetic chromogenic TAL / LAL for endotoxin quantitative for 35 years. We are the major TAL reagent provider in China. Our clients are biological products manufacturers, intravenous (IV) fluids manufacturers and injectable drug providers. Most of the pharmaceutical plants at China are our clients.
1. Product information
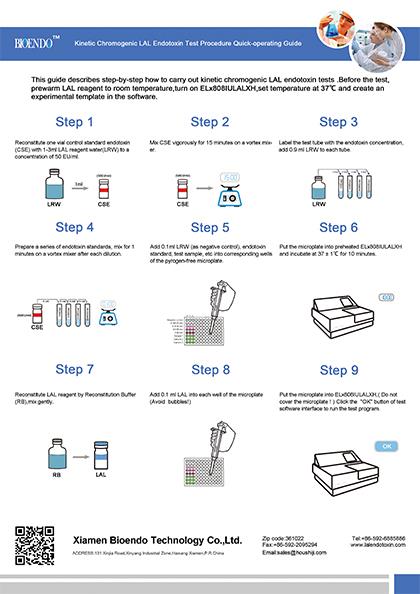
Kinetic Chromogenic LAL assay is the most sensitive LAL assay for endotoxin test (pyrogen detection). In kinetic chromogenic method, LAL reagent is co-lyophilized with chromogenic substrate. As a kinetic lal test, a kinetic instrument like an incubating kinetic microplate reader or kinetic tube reader is required. Same as the kinetic turbidimetric LAL reagent, we recommend the kinetic chromogenic assay performs in an incubating microplate reader Elx808IULALXH. The increase of OD at 405nm is recorded and interpreted by the instrument and software, the endotoxin concentration is also calculated. Kinetic chromogenic assay combines the advantages of kinetic turbidimetric and end-point chromogenic method. The detection limit of kinetic chromogenic assay is 0.005EU/ml to 50EU/ml. Without depending on the activity of clotting protein to form the gel clot, the assay is strong resistance to interference. Especially suitable for the biological samples, such as a vaccine, antibody, protein, nucleic acid, clinical samples analysis of bacterial endotoxin.
2. Product parameter
Assay range: 0.005-50EU/ml
1.7ml or 2.2ml per vial
3. Product feature and application
Strong resistance to interference. Recommended to endotoxin detection of biological products, such as vaccine, antibody, protein, nucleic acid, clinical samples.
Operation chart
Standard curve :
4. Ordering information
Catalog Number | ml/vial | Tests/vial | Vials/pack |
KC05 | 0.5 | 4 | 10 |
KC11 | 1.1 | 10 | |
KC17 | 1.7 | 15 | |
KC22 | 2.2 | 20 | |
KC28 | 2.8 | 26 | |
KC52 | 5.2 | 50 |
5. Product Qualification

6. Ordering FAQ
Product condition:
The LAL reagent sensitivity and the Control Standard Endotoxin potency are assayed against USP Reference Standard Endotoxin. The LAL reagent kits come with product instruction, Certificate of Analysis, MSDS.
How to order:
Please contact sales@houshiji.com to order the LAL reagents endotoxin detection kit. There is no minimal order volume required. The price depends on the order volume. We provide distributor discount for our distributors. The price is in US dollars. The final cost will be the order price plus the shipping cost. We need to know the shipping item to calculate the shipping weight.
Payment Method:
We required 100% prepayment for all orders. We accept T/T bank transfer or Paypal. If pay by Paypal 5% additional charge is required.
Shipping Condition:
Once we received the payment, we will ship in two days. Shipping is usually by air, it takes less than one week to reach the location. LAL Reagent is stable at controlled room temperature for more than two weeks. We usually ship the reagent at room temperature. After receipting LAL Reagent, long term storage at 2-8? is required.
Storage Condition:
The LAL reagent is stable for more than two years at 2-8?. Store LAL reagent at 2-8? , avoid light. LAL Reagent Water is stable for more than three years at 2-20? .
Note:
LAL Reagent ( TAL Reagent ) from our company are formulated from the aqueous extract of circulating amebocytes of Chinese horseshoe crab (Tachypleus tridentatus).
Payment & Shipping

More Product
Recommend Product